You are about to leave this site.
Please be aware that your current cart is not saved yet and cannot be restored on the new site nor when you come back. If you want to save your cart please login in into your account.

Cell Identity
Lab Academy
- Cell Biology
- Cell Culture
- Cell Identity
- CO2 Incubators
- Cell Culture Consumables
- Essay
According to ICLAC (International Cell Line Authentication Committee) terms of reference, a cell line is considered to be misidentified if its DNA profile (genotype) is no longer consistent with the donor from whom it was first established.
Causes of misidentified cell lines
Cross-contamination is not always the reason for an altered genotype of a cell line. Cells in culture can also change over time without any external contamination. Cell line genetics may be altered by chromosomal duplications, deletions or rearrangements, mutations and epigenetic changes [5]. Genomic rearrangements can also result from many types of stresses such as contamination with bacteria, mycoplasma or exposure to drugs [6].
Cross-contamination is not always the reason for an altered genotype of a cell line. Cells in culture can also change over time without any external contamination. Cell line genetics may be altered by chromosomal duplications, deletions or rearrangements, mutations and epigenetic changes [5]. Genomic rearrangements can also result from many types of stresses such as contamination with bacteria, mycoplasma or exposure to drugs [6].
Consequences of working with the wrong cell line
What can you do to ensure working with the right cells?
What/ Who is ICLAC (International Cell Line Authentication Committee)?
Goal 1:
Make Cell Line Misidentification More Visible
ICLAC members have developed a database of misidentified cell lines. This database is accessible for the scientific community and is regularly updated. The ICLAC list of misidentified cell lines can be found on the ICLAC website.
Goal 2:
Promote Authentication Testing
ICLAC also focusses on scientific community education. On their website, documents (guidelines, checklists, protocols, etc.) are available to help scientists learn about the topic of cell identification and to facilitate the integration of authentication within good cell culture practices. The group also offers assistance to researchers wishing to authenticate cell lines. Finally, ICLAC members closely interact with journals and funding bodies to encourage mandatory authentication testing.
Incorporating cell authentication testing into cell culture routine
Whereas no mandatory testing schedule exists at this time, it is recommended to systematically check and document cell line identity at least:- When a foreign cell line enters the cell culture laboratory.
- During establishment of frozen cell stocks.
- At the beginning and at the end of an experimental project.
- Before distributing a cell line.
Choosing a cell authentication method
Several different methods are available to detect inter-species and intra-species cross-contamination in cell cultures. These methods verify cell line identity on the protein or genetic level. The acquisition and application of authentication methods is not difficult for trained scientists. However, reliable interpretation of the results requires advanced experience. Depending on the testing method, special laboratory equipment might also be necessary. For support, commercial services may be employed to perform testing and/or interpretation of analysis within a short turnaround time.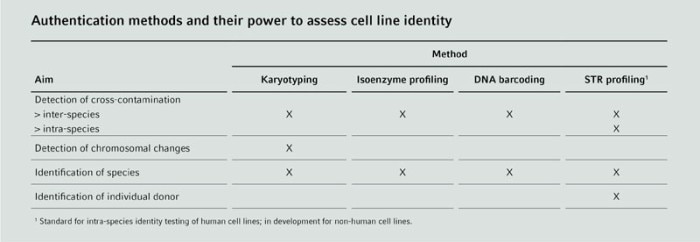
1. http://iclac.org/wp-content/uploads/ICLAC_Terms-of-Reference_2015_v2_6.pdf
2. Gartler (1967) Genetic markers as tracers in cell culture. Natl Cancer Inst Monogr. 26:167-95.
3. Gartler (1968) Apparent HeLa cell contamination of human heteroploid cell lines. Nature. 217:750-751.
4. Nelson-Rees and Flandermeyer (1977). Inter- and intraspecies contamination of human breast tumor cells HBC and BrCa5 and other cell cultures. Science. 195(4284):1343-4.
5. Lorsch et al. (2014) Cell Biology. Fixing problems with cell lines. Science. 346(6216):1452-3.
6. Marx (2014) Cell-line authentication demystified. Nature Methods. 11: 483-488.
7. http://www.celllineauthentication.com/journal-requirements.html
8. http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-103.html
9. Freedman et al. (2015) The culture of cell culture practices and authentication - Results from a 2015 survey. BioTechniques. 59:189-192.
STR profiling
References:
1. Almeida et al. (2014) Mouse cell line authentication. Cytotechnology. 66(1): 133-47.
2. Almeida et al. (2011) Authentication of African green monkey cell lines using human short tandem repeat markers. BMC biotechnology. 11:102.
Karyotyping
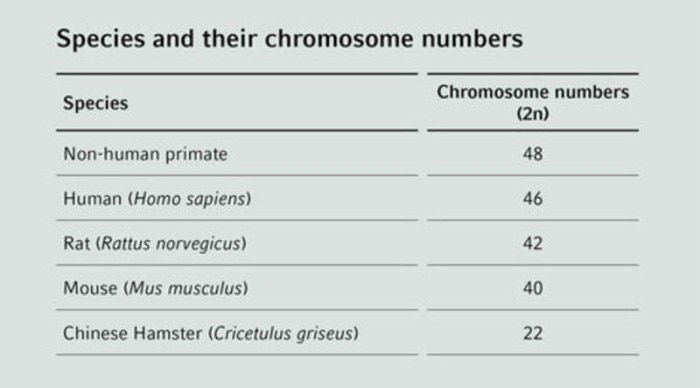
Chromosomes provide information about species, gender and chromosomal integrity and are characterized by their species-specific number and structure. Since these parameters can differ strongly between different species, the illustration of chromosomes in the form of a karyogram can provide information about the origin of cells and can often be related back to a specific species. The most prevalent cell lines used in cell cultures derived from human, primate, rat, mouse or hamster can be distinguished by their chromosome number. Thus, karyotyping is essentially used for detection of inter-species cross-contamination. If a detailed karyotype analysis of the original material is available, cytogenetic analysis may be used for the analysis of individual cell lines in order to determine whether intra-species cross-contamination has occurred.
For the purpose of a karyogram, mitotic cells are arrested in the metaphase of the cell cycle when chromosomes assume their most condensed confirmation. Subsequent staining and microscopic analysis will reveal characteristic features. In order to obtain representative data, this technique must be established for each individual cell line. In addition to its labor-intensive and time-consuming aspects, karyotyping requires extensive laboratory experience within the cytogenetic field in order to ensure reliable interpretation. This is especially true in cases of intra-species cross-contamination. In particular the use of transformed cells with incidences of aneuploidy/heteroploidy (loss or gain of chromosomes), might hinder species identification without using specific markers in advanced analyses.
Isoenzyme profiling
In most cases, the analysis of four different isoenzymes (nucleoside phosphorylase, glucose-6-phosphate dehydrogenase, malate dehydrogenase, lactate dehydrogenase) is sufficient to confirm the identity of the sample of interest [1]. Only the differentiation of mouse and hamster cell lines requires the additional analysis of peptidase B. In general, isoenzyme profiling is suggested as a rapid and easily conducted technique to check for inter-species cross-contamination. It can be performed with a commercially available kit designed to confirm authentication by determination of the mobility of seven isozymes for six cell lines in less than three hours [2].
References:
1. Nims et al. (1998) Sensitivity of isoenzyme analysis for the detection of interspecies cell line cross-contamination. In vitro Cell Dev Biol Anim. 34: 35-39.
2. Hay et al. (2000) Cell line preservation and characterization. In Masters, J.R.W (ed) Animal cell culture, a practical approach. Oxford, U.K., IRL Press at Oxford University Press. 95-148.
